The task of differentiating between Extracellular Vesicles (EVs) and other nanoparticles (e.g. Nanobubbles, protein aggregates, inorganic salt precipitates) typically found within a biological sample can be very challenging.
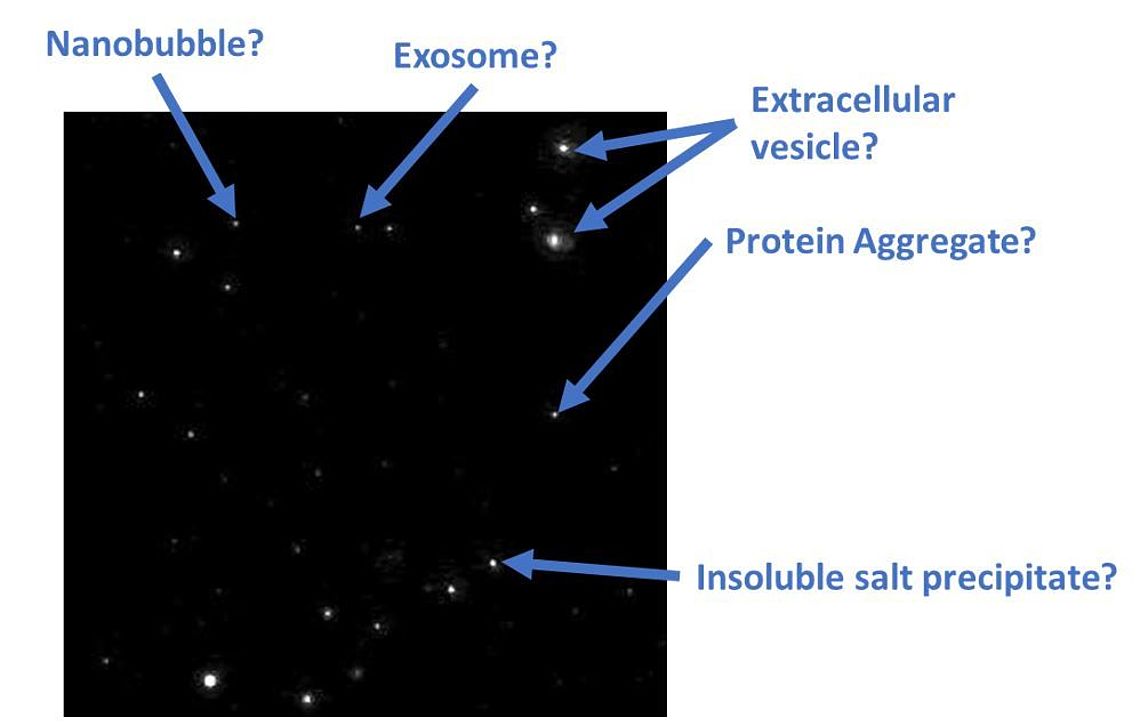
Figure 1: Identification problem of particles in a classical scatter NTA video derived from an EV preparation
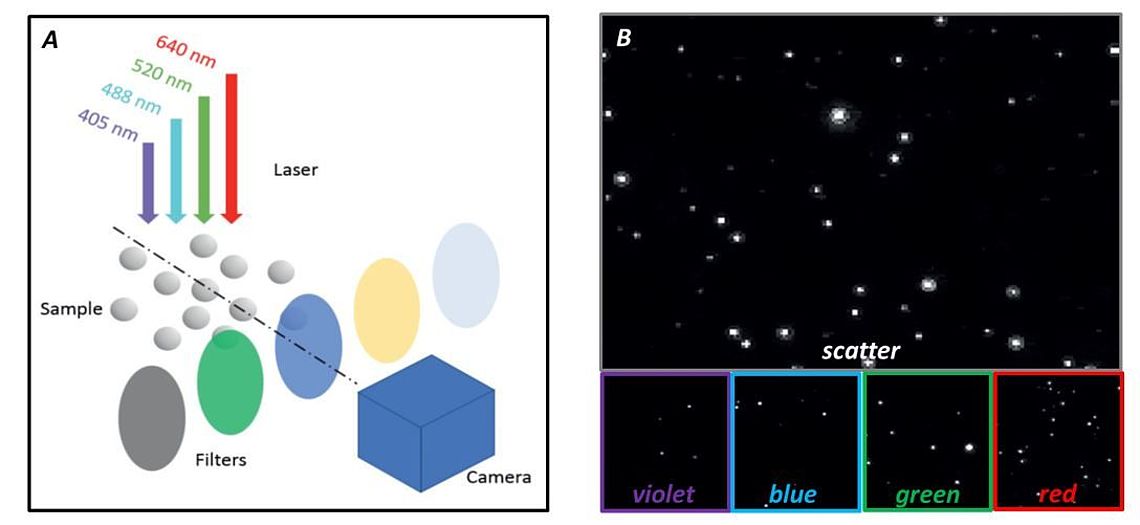
Figure 2: Measurement modes of ZetaView® QUATT A) Scheme of the optical laser and filter arrangement B) Example of five channel EV measurement
Here we report a unique technology for rapid determination of membranous particles along with an analysis of multiple EV biomarkers. In spite of the experimental sophistication, the operational costs of our instrument are kept to a minimum.
NTA is a well-established method to analyze the size & concentration of EVs with sizes ranging between 50 – 200 nm (Giebel and Helmbrecht, 2017; Soo et al. 2012). Unfortunately, the standard scattered-light methodology is not able to discriminate EVs from other ultrafine particles in the same size range (see figure 1).
To overcome the limitations of standard NTA, we developed a series of NTA instruments which are optimized not only for scatter light but also for fluorescence NTA (Melzer et al., 2019). Each NTA system is equipped with up to four excitation lasers along with their corresponding emission filters. Our multi-laser systems are driven by software that provides rapid and automated exchange between: (i) the detection modes (scatter vs. fluorescence), (ii) the different laser sources, and (iii) the corresponding emission filters (See instrument page).
Our instruments make it possible to measure the size, concentration, zeta potential, and fluorescence intensity (up to four detection channels), all within a single sample volume (figure 2). Furthermore, our new patent-pending anti-bleaching technology allows the use of fast bleaching fluorophores like e.g. FITC and Calcin.
Applications:
- Purity check of Extracellular Vesicles preparations
- Identification of Exosomes in Extracellular Vesicle preparations with tetraspanines
- Identification of platelet-derived exosomes
- Identification of tumor-derived exosomes
- Expression control of GFP-tagged proteins in Extracellular Vesicles
- Identification of intravesicular DNA / RNA containing Extracellular Vesicles with DAPI
- 4-color immunophenotyping of Extracellular Vesicles
Literature:
Giebel B, Helmbrecht C. Methods to Analyze EVs; Methods Mol Biol. 2017;1545:1-20.
Soo C.Y., Song Y., Zheng Y., Campbell E.C., Riches A.C., Gunn-Moore F. and Powis S.J. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012, 136:192–197. doi:10.1111/j.1365-2567.2012.03569.
Melzer C, Rehn V, Yang Y, Bähre H, von der Ohe J, Hass R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers (Basel). 2019 Jun 9;11(6). pii: E798. doi: 10.3390/cancers11060798.
